1. Association of predinisone and antimalarials and echocardiographic findings in asymptomatic cardiovascular patients with SLE.
Jochims, I; Mota, LHM; Muniz, L; Vasconcelos DF; Santos-Neto, LL; University of Brasilia
Introduction: Systemic lupus erythematosus (SLE) is an autoimmune inflammatory disease that presents with increase of cardiovascular risk. Echocardiogram can detect morphofunctional cardiac changes and predict clinical outcomes in patients with SLE¹.
Objectives: To evaluate echocardiographic morphofunctional parameters in women with SLE, using conventional echocardiogram and to relate the echocardiographic findings to disease-related factors and therapeutics.
Methods: We have selected 51 women with SLE, without cardiovascular symptoms, under regular medical follow-up. Patients who had limitations to do echocardiography, smokers, and those with a creatinine level higher than 1.5mg/dL were excluded. 51 patients were divided into two groups, patients with SLEDAI < 6 (n=30) and with SLEDAI≥6 (n=21). They were submitted to clinical evaluation, laboratory tests and a traditional echocardiogram.
Results: Patients presented an average age of 34.5 years and average time of diagnosis of SLE of 7.2 years. In the comparison between groups, patients with SLEDAI≥6 had a higher daily dose of prednisone (p=0.0016), more hospitalizations in the last 12 months (p=0.0173), and a higher cumulative dose of pulse therapy with methylprednisolone (p=0.008). Patients with SLEDAI < 6 had a longer average time of antimalarials (AM) use (p=0.0309). Regarding the echocardiographic parameters, group with SLEDAI≥ 6 presented greater left ventricular mass (LVM, p=0.0156), thickness of ventricular septum (p=0.0106) and left ventricular posterior wall (LVPW, p=0.0273). In multivariate analysis the LVM presented a positive association with age (p=0.0160), current daily dose of prednisone (p=0.0009) and time of AM use (p=0.0026), r² 0.3625. Regarding the thickness of the interventricular septum, there was a positive association with age (p< 0.0001), current dose of prednisone (p< 0.0001), SLEDAI (p=0.02), SLICC (p=0.0093) and pulse with methylprednisolone (p=0.0062), with r² 0.6983. There is a positive association of daily dose of predinisone with the parameters LVPW, LVM and septum thickness and AM was a predictor of greater LVM.
Conclusion: Several factors may contribute to cardiac morphofunctional changes in SLE. Ventricular hypertrophy in asymptomatic cardiovascular patients was not related to the use of prednisone or time of AM use in other studies, however these factors should be taken into account. There are no adequate study designs in the literature to evaluate the effect of high doses of corticosteroids and time of AM use on cardiac morphology and function. AM induced cardiomyopathy is a rare, probably under-recognized, complication of prolonged AM treatment, presents as a hypertrophic, restrictive cardiomyopathy with or without conduction abnormalities. Early recognition and drug withdrawal are critical with a survival rate of almost 55%². Longitudinal studies are needed to determine the effect of prednisone and AM use in subclinical echocardiographic findings to avoid unfavorable cardiovascular outcomes.
References:
¹ Chen J, et al. Heart involvement in systemic lupus erythematosus:a systemic review and meta-analysis. Clin Rheumatol 2016;35:2437–48;
² Tselios K, et al. Antimalarial-induced cardiomyopathy: a systematic review of the literature. Lupus 2017; 0: 1-9.
2. Disease activity state in patients with rheumatoid arthritis included in the biobadabrasil registry
I. M. M. Laurindo* 1, M. Pinheiro1, J. Macieira1, A. Duarte1, B. Kahlow1, R. Ranza1, J. Miranda1, M. Bertolo1, V. Valim1, G. Castro1, M. D. F. Lobato1, D. Titton1, R. Teodoro1, J. Moraes1, C. Brenol1, I. Costa1, V. Fernandes1, H. Carvalho1, I. Pereira1, W. Bianchi1, A. Hayata1, P. Louzada1, A. Kakehasi1, R. Toledo1, G. Castelar1, I. Silva1, A. Ranzoline1, G. Christopoulos1 on behalf of BIOBADABRASIL
1Brazilian Register of Biological Agents in Rheumatic Diseases, São Paulo, Brazil
Objectives: to analyze disease activity of RA patients at the start of treatment with a biologic agent (adalimumab, infliximab, certolizumab, etanercept, golimumab, abatacept, rituximab and tocilizumab -bDMARDs), target (tofacitinib-tsDMARDs) therapy or conventional DMARDs (comparison cohort of RA patients – csDMARDs).
Methods: BiobadaBrasil is a prospective observational cohort study, part of an international initiative (biobadaAmerica) in collaboration with BiobadaSeR; established in 2009, is an ongoing study supported by the Brazilian Society of Rheumatology (29 public centers located at academic instituitions and 2 in private practice) focused on the study of adverse events in patients on biologic therapy, but, by decision of its investigators, disease activity scores were also recorded at the beginning of each treatment. Sex, age, disease duration, RA -DAS-28, concomitant treatments at baseline were collected. Results expressed in mean±SD, %(n)
Results: Results – Data from 1984 RA patients (67% on ts/bDMARDs) were analyzed: 86% of the patients were women; mean age=56.6±14.95yrs; disease duration=14.4±9.42; 87% RF positive. There were 3802 treatment courses, 67% with aTNF (Adalimumab-ADA, Certolizumab-CTZ, Etanercept-ETA, Golimumab-GOL, Infliximab-INFL), 33% non-aTNF (Abatacept-ABA, Rituximab-RIT, Tocilizumab-TCL) including Tofacitinib-TOF. The table below depicted the medications prescribed at the initiation of a new treatment course:
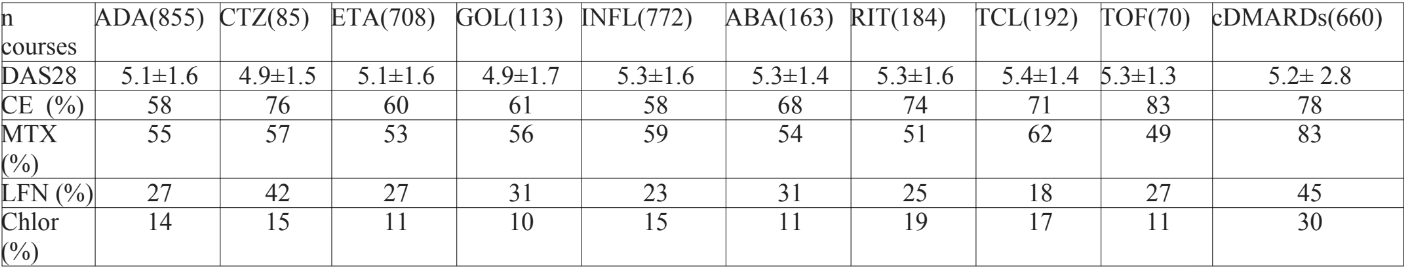
Conclusions: Conclusion: patterns of bDMARDS and csDMARDS use clearly surfaced not always in accordance to guidelines. Extensive use of corticosteroids is identified as well as reduced proportion of association of MTX with biologic agents. Possibilities of improvement clearly needed to be addressed.
Acknowledgements: for data monitoring P Cabral; contributing to BiobadaBrasil registry, R. Giorgi, H. Pereira, M.Scheinberg, F.Sztajnbok, W.Vieira.
3. Biological drugs in the treatment of rheumatoid arthritis: real life data in a Brazilian multicentric study
A. Gomides1,* G. Pinheiro2, A. Santos2, C. Albuquerque3, R. Giorgi4, S. Radominski5, I. Pereira6, M. Guimarães7, M. Bértolo8, P. L. Júnior9, K. Bonfiglioli10, C. Brenol11, M. Cunha12, L. Mota3
1Universidade de Brasília, UnB- Brasil, Brasília, 21. Universidade do Estado do Rio de Janeiro, Rio de Janeiro,3 Universidade de Brasília, Brasília, 43. Instituto de Assistência Médica ao Servidor Público Estadual, São Paulo, 54. Universidade Federal do Paraná, Curitiba, 65. Universidade Federal de Santa Catarina, Florianópolis, 76. Universidade Federal de Minas Gerais, Belo Horizonte, 87. Universidade Estadual de Campinas, Campinas, 98. Universidade de São Paulo – Ribeirão Preto, Ribeirão Preto, 1010. Universidade de São Paulo, São Paulo, 1111. Universidade Federal do Rio Grande do Sul, Porto Alegre, 129. Universidade Federal do Pará, Belém, Brazil.
Background: Rheumatoid arthritis (RA) is a chronic disease, characterized by inflammatory involvement of the synovial joints. The “treat to target” concept is well established in the rheumatologic community, however, in many patients, especially in developing countries, its implementation is not feasible. Considering the high costs of treatment com of RA and the limited national epidemiological data available on this disease, we sought to describe the profile of use of biological drugs in Brazilian patients with RA to help the decision-making process by public health managers. Objectives: To describe the frequency and time of use of biological drugs in Brazilian patients with rheumatoid arthritis.
Methods: The REAL – RA in real life in Brazil – is a multicenter prospective cohort study, with twelve-month follow-up period. To be included in this study, consecutive patients from 11 tertiary rheumatology centers had to meet the 1987 ACR or the 2010 ACR/European League Against Rheumatism (EULAR) criteria. Data were collected during routine clinical care and previous medical records were used as secondary sources. The present study present data taken from the participants’ initial assessment. This research was approved by the Ethics Committees of each center. Results: A total of 1125 patients were analyzed. 89% were women with a mean age of 56.6 years. The main clinic data were: DAS 28 (median) = 3.52, HAQ (median) = 0.87 and CDAI (median) = 9. 1022 (90.84%) used synthetic DMARDs and 406 (36.09%) biologic therapy. The frequency of use of the biologic therapy was: abatacept (73 patients / 6.49%), etanercept (66 / 5.87%), tocilizumab (60 / 5.33%), adalimumab (54/4.8%), infliximab (50 / 4.44%), rituximab (49 / 4.36%), golimumab (37 / 3.29%), certolizumab (17, 1.51%). The time of use of the biological drugs is presented in table 1.
TABLE 1: Time (in years) of use of biological drugs in patients with rheumatoid arthritis
| DRUG | MEAN | MAXIMUM |
| ABATACEPT | 1.95 | 8 |
| ADALIMUMAB | 1.70 | 12 |
| CERTOLIZUMAB | 0.63 | 2.0 |
| ETANERCEPT | 1.49 | 9.0 |
| GOLIMUMAB | 0.65 | 2.0 |
| INFLIXIMAB | 1.56 | 9.0 |
| RITUXIMAB | 1.27 | 6.0 |
| TOCILIZUMABE | 2.0 |
6.0 |
Conclusions: The therapeutic profile of this cohort of Brazilian RA patients shows some interesting results. The relatively high number of patients on biologics, compared to other studies, may be related to fact that the centers involved were reference centers, probably dealing with more difficult cases.
References:
1) Azevedo, AB; Ferraz, MB; Ciconelli, RM. Indirect Costs of Rheumatoid Arthritis in Brazil. Value in Health 2008;11 (5):869-877
2) Boonen, A & Severens, JL. The burden of illness of rheumatoid arthritis. Clin Rheumatol 2011; 30 Suppl 1:S3-S8.
(DOI:10.1136/annrheumdis-2018-eular.709)
4. Factors associated with functional capacity in a brazilian cohort of patients with rheumatoid arthritis: results from the “real” study
1. A.A.V. Pugliesi1, 2. E.D.A. Macedo1, 3. M.B. Bertolo1, 4. R. Giorgi2, 5. S. Radominski3, 6. I. Pereira4, 7. M.F. Guimaraes5, 8. P. Louzada6, 9. M.D.F. Cunha7, 10. K. Bonfiglioli8, 11. C. Brenol9, 12. L.M.H. Mota10, 13. G. Castelar-Pinheiro11
Background: Rheumatoid arthritis (RA) is associated with impairments in functionality, affecting aspects such as physical capacity, independence, mental health, social and professional life. Health Assessment Questionnaire-Disability Index (HAQ-DI) is a validated tool for assessing functional capacity in RA patients. A simple questionnaire with a score of 0 to 3 is applied, with an inverse relationship between grade and functionality. Previous studies have shown worse functional indexes in patients with high disease activity and established joint damage.
Objectives: To relate clinical, laboratory and therapeutic aspects with HAQ-DI in a large cohort of Brazilian patients.
Methods: A prospective, multicenter cohort study (“REAL” Study) involving 11 Brazilian centres specialised in the treatment of RA patients. All patients were submitted to at least 3 clinical evaluations in a 12 month period. Only patients older than 18 years and classified as RA according to 1987 (ACR) or 2010 (ACR/EULAR) criteria were evaluated. HAQ-DI was applied for assessing functional capacity, and the results were analysed for association with clinical, laboratory and therapeutic elements. Comparison between groups was performed using Mann-Whitney or Kruskal-Wallis tests.
Results: Overall, 1116 patients (89.43% females, mean age 58±11 years) took part in the study. Rheumatoid factor (RF) in high levels and bone erosion were both associated with higher HAQ-DI indexes (p: 0.0244 and p< 0.0001, respectively). Of all patients, 89.7% were using conventional synthetic disease-modifying antirheumatic drugs (DMARDs) and 36.5% were on biologic DMARDs or targeted-synthetic DMARDs. The use of any conventional synthetic DMARD was associated with lower HAQ-DI indices (p: 0.0243), while the use of any biologic DMARD or targeted-synthetic DMARD was related to greater functional impairment (p: 0.0018). By evaluating separately, abatacept (p: 0.0046), rituximab (p: 0.0001) and tocilizumab (p: 0.0441) were associated with higher levels of HAQ-DI. The results are summarised in table 1.
Conclusions: In our prospective cohort, patients with high levels of RF, bone erosion, in use of any biologic DMARD or targeted-synthetic DMARD, abatacept, rituximab or tocilizumab had worse functional capacity indexes. When compared to non-use, the use of any conventional DMARD was associated with better rates of HAQ-DI.
5. BONFIGLIOLI, K. ; CARRICO, H. ; Mota, L ; SANTOS, A. B. V. ; ALBUQUERQUE, C. ; GIORGI, R. ; RADOMINSKI, S. ; PEREIRA, I. ; GUIMARAES, M. F. ; BERTOLO, M. ; LOUZADA, P. ; CUNHA, M. F. ; BRENOL, C. ; CASTELAR-PINHEIRO, G. . Extra-articular manifestations in rheumatoid arthritis: a comprehensive analysis in a large cohort. In: Annual European Congress of Rheumatology- EULAR 2018, 2018, Amsterdam. Annals of the Rheumatic Diseases, 2018. v. 77. p. A301-A301.





